Results for 'post traumatic stress disorder'

Leading From the Front: 3 Leadership Lessons From Mark Cuban
Dec 17th • 6 mins read

Leadership Lab: 10 Ways Executives Can Stay Visible, Valuable Between Jobs
Oct 22nd • 5 mins read

Leadership Lab: Take a Cue From Athletes by Working With an Executive Coach
Feb 19th • 6 mins read


Leadership Lab: 4 Ways Biopharma Leaders Can Prepare for Media Interviews
Aug 20th • 6 mins read


Practical Advice for When You're Downsized. What Activities will Pay-off after a Lay-off?
Sep 15th • 5 mins read

New Book Unites Oncology’s Brightest Minds To Innovate Cancer Cures
Sep 9th • 5 mins read



MSL Hiring and Recruitment: 5 Ways to Support Diversity and Inclusion
May 19th • 2 mins read


Combatting the “Summer Slow Down" – MSL Job Search Tips for Slower Months
Jun 23rd • 2 mins read


Accelerated drug approvals in oncology: Pros and cons
Sep 14th • 4 mins read
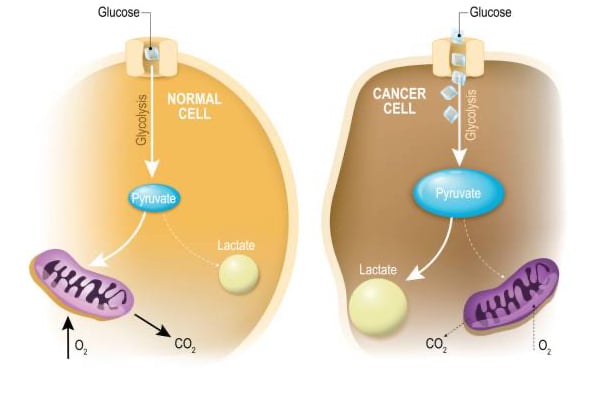
“Oncometabolism: The switchboard of cancer: An editorial”
Feb 1st • 1 min read
Confounding factors in exposure–response analyses and mitigation strategies for monoclonal antibodies in oncology
Nov 20th • 12 mins read

Advances and Challenges in Pediatric and Childhood Cancers
Jun 27th • 2 mins read

Comment on: Oncology research in Saudi Arabia over a 10-year period. A synopsis
Jun 24th • 3 mins read
